Pfizer’s COVID-19 vaccine received a vote of confidence from US Food and Drug Administration (FDA) advisers late on Thursday (December 10, 2020, early Friday in the UAE), clearing the way for the agency to authorise its use.
US media reported that the FDA approval of a COVID-19 vaccine is likely “within days”.
Highlights
- The panel of experts voted in favour of emergency authorisation for people 16 and older.
- The FDA does not have to follow the advice, but it usually does.
- The authorisation is expected within days, paving the way for healthcare workers and nursing home residents to begin getting shots next week.
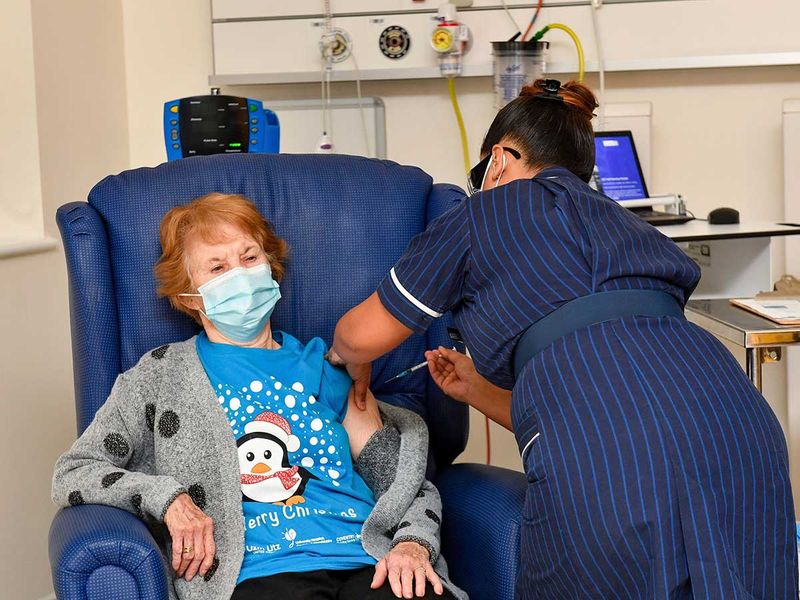
Image Credit: AP

